Chemistry Assignment: Analyse The Concentration Of Hydrogen Ion
Question
Task:
Your report must be divided into the following sections:
1. Present equations that define: (a) the pH of a solution; and (b) the generic meaning of p (as in pH).
2. Discuss the concept of dissociation of weak acids; present the equations that define the acid dissociation constant (Ka) and the pKa.
3. Explain the essential chemical components for preparing a pH buffered solution.
4. Explain the generic principles of acid-base titration.
5. Explain the concept of buffer capacity and present the equation that defines the quantitative measure ß.
6. Explain why buffer solutions exhibit maximum buffering capacity at a particular pH.
7. Identify the dominant buffering components of phosphate buffer (pH 7) and report the applicable pKa value at that pH.
8. Discuss the autoionization of water, the relationship pH + pOH = 14, and report the concentration of the hydroxide ion (OH- ) in water at 25 °C.
9. Explain the meaning of the expression ‘strong base’.
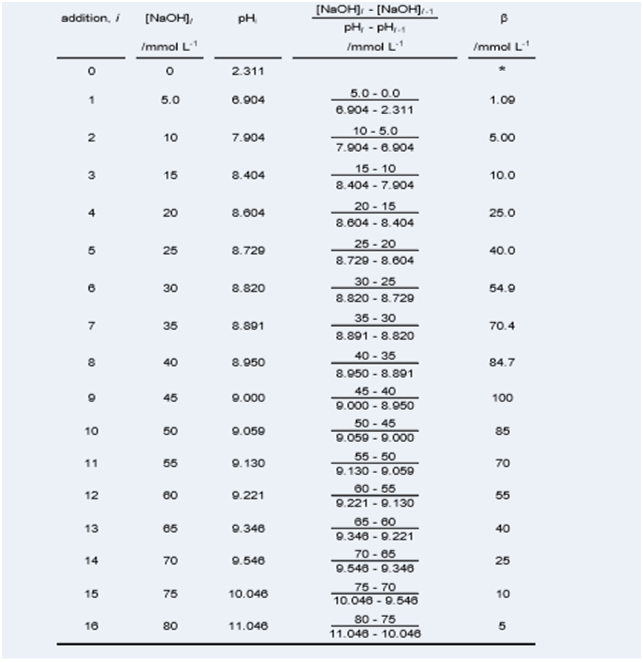
10. Using the example data in the table below, plot the buffer capacity estimates in a single scatter plot (buffer capacity on the vertical axis, measured pH on the horizontal axis).
Copy and paste the plot into your report. Give the figure: a figure number; a short descriptive title; and the minimum necessary information. Precede the figure with text that describes the important features of the buffer capacity curves and refers to the scatter plot by citing the figure number.
Chemistry and Molecular Genetics
In a chemical reaction, unionized molecules and the ions in the solution of weak electrolytes established an equilibrium known as ionic equilibrium. While studying the equilibrium between acid and base in a solution we analyse the concentration of hydrogen ion present. If we mix a weak base and its conjugate acid or vice versa in water then it will acts as a buffer solution.
pH = -log [H+] here, [H+] symbolizesconcentration of hydrogen ion which comes from acid.pH can be acidic, basic and neutral.
The standard meaning of p [Asin pH] is power of hydrogen. Generally H in pH presents Hydrogen but some exceptions we use p which means power.
An acid which does not dissociate completely in a solution is a weak acid that signifies it does not donate all hydrogen in solution. Example like acetic acid or vinegar.
A Weak acid like CH3COOH dissociates in CH3COO- and H+
Then, degree of dissociation = kac
Here, ka = Dissociation constant
C = concentration
pka = - logka
pka and ka are inversely proportional to each other .
The value of degree of dissociation is affected by following factors:
a) Ostwald's Dilution Law:
On diluting the solution, concentration decreases which ultimately leads to increase in degree of dissociation.
b) Temperature:
We know, dissociation is an endothermic Reaction i.e. H= +ve (On increasing the temperature Keqincreases).
As Keq increases, concentration of product increases. Ultimately degree of dissociation increases.
pH buffered solution is the combination of a weak acid and its conjugate base.In general, CH3COOH and sodium acetate is used as a pH buffered solution. Add 5.772 g ofSodium acetate with 1.778g of acetic acid and prepare solution with distilled water.
ThenpH is adjusted by using HCL.
Otherwise a pair of NH3(weak acid) and NH4OH (conjugate weak base) will be used.
General principle of acid – Base titration is that acid and base will be added together henceforth they give neutralisationreaction .This reaction change the pH of solution and solution convert into neutral pH.
Acid reacts with base with adding suitable indicator like phenolphthaleinto find end point colourless to pink and find unknown concentration of acid by using M1V1 = M2V2.
Here, M2 = Molarity of unknown acid
M1 = Molarity of known acid
V1= Volume of acid used
V2 = Volume of base used (from experiment)
It is a quantitative evaluationfor the determination of concentration of an acid and base by neutralizing the solution with standard solution of base and acid of unknown concentration. A pH indicator used to determine the progress of the reaction.
Indicators like Phenolphthalein(Colourless to pink), Methyl orange (Red to yellow colour) etc.
The concept of buffer capacity means, efficiency of buffer with resistance to pH change.In buffer, solution can repel a slight amount of change in pH on adding acid or alkali in solution.
ß = dn / dpH , n= no of equivalents of strong base added. The Buffer capacity enumerates the ability of solution to resist changes in pH by either absorbing [H+] or [OH-] ions.
At a particular pH, buffer capacity of buffer solution reaches to maximumbecause it will be easy for solution to resist the change in pH. Ratio of 1:1 is the best amount for preparing a buffer solution.
pH = pKa
Concentrations of the acidic components isequal to concentration of basic components of buffer system.It is used to measure the power to repel change in pH of Buffer Solution and the number of moles of the acid or Base which when addedto 1 litre of buffer solution, change in pH will be 1.
Any solution will behave as a buffer solution only when the ratio of concentration of salt to base or acid is from 1 to 10.
pH = pKa+ log
pH = pKa +1
pH = pKa-1
Generally phosphate buffer have wide range due to phosphoric acid having three dissociation constants (triprotic acid in nature) allow formation of buffer each of the pH range of 2.14, 6.85, and 12.31.
Phosphate buffer is the highly soluble and buffering capacity. Phosphate buffer breaks in three different steps having three different ka values
Reaction involve:H3PO4 -----H2PO4-ka1
H2PO4- -------HPO42- ka2
HPO42- ---------PO43-ka3
pH = Pka1 + Pka2 / 2
and, (HPO42-), pH = ka2+ ka3/ 2
(H2PO4-), pH = ka1 + ka2 / 2
As dissociation of water is an endothermic reaction. So increase in temperature leads to increase in kw.
At 25°C,
pH =7 pOH ( Neutral solution)
pH< 7 or pOH > 7 (acidic solution)
pH > 7 or pOH < 7 (Basic solution)
Ionic product of water in equilibrium is steady so it depends on temperature.
Hence, ionic product of water will be a constant alwayswhatsoever will be dissolved in water.
Kw = [H+] [OH-] (here, kw= ionic product of water)
For pure water, [H+] = [OH-]
[H+] = [OH-] = 10-14
At 25°C, kw = 10-14
kw= [H+]2
10-14= [H+]2
[H+] = 10-7
-log [H+] = - log10-7
pH = 7 (Neutral)
Ka x 55.55 = Kw
But different temperature
At 29°C, pH = 6 (neutral)
A strong base refers to that base which dissociates 100% in aqueous solution. Those base which react with water molecule and give one and more hydroxide ion. The OH- ionsconcentration in strongly basic solution is identical to undissociated base. Example, the OH- of alkali metals like NaOH and Ca(OH)2. Strong base capable of elimination of hydrogen from weak acids, strong base can eliminate the hydrogen of weak acid C-H in deficiency of H20pH of a strong base.
Kw = [H+] [OH-]
-log kw = - log [H+] + (-log [OH-] )
pkw = pH +pOH
at 25°C, pH + pOH = 14
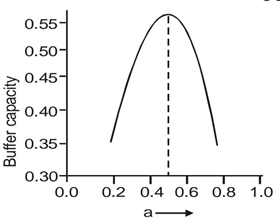
Graph between buffer capacity and pH (denoted by a) shown in figure 10.According to data, graph will continously increases and at certain peak gets decreasing due to decrease buffer capacity and increasing pH value. Initially buffer capacity increase because ions form highly but at one stage ions get diffused and buffer capacity gets decreases. A buffer is capacity to repeldeviations in pH when minoramounts of acid and base are added into solution. The capability of buffer to engross acid or base by look at how buffer repels changes pH.
Strong acids are those acids which are 100% ionized in contrast weak acids are partly ionized. As the hydrogen ion concentration increases pH decreases. To cling on to the pH at nearly constant value buffer solution is used.In buffer, solution can repel a slight amount of change in pH on adding acid or alkali in solution.