Risk Management Plan Assignment: Identification Of Risk Factors In The Usage Of Tablets
Question
Task: Deliver a report on risk management plan assignment that includes a risk management plan in order to identify the risk factors in the usage of tables.
Answer
Introduction
The following risk management plan assignment revolves around the concept of ‘Risk management’ which is termed as a crucial part of ‘project management plan’ and helps project aspects to enable the facilities of information alignment along with the fulfillment of the essential criteria (Lin et al., 2017). It is essential for a project to be completed with the alignment of risk mitigation process that the negative impacts of the harmful factors can be minimized. ‘Risk management plan’ leads a project towards achieving goals and objectives without getting obstructed through any negative impacts of the aligned risk factors. The main aim of the ‘risk management plan’ is to protect the project aspects from getting enlisted into unsuccessful criteria with the information alignment that helps a project to enable the effectiveness of project management plan. The following project examined in the risk management plan assignment would involve a risk management plan for identifying the risk factors in the usage of tablets. The plan would also include some mitigation on how to reduce the negative impacts of the tablets and its coating. A risk assessment matrix would be provided to understand the possible impacts of the risk assessment plan.
Risk Identification
Tablets are the immersive parts of human life as these are helpful aspects to cure pain or disease. People take a sufficient number of tablets to live a healthy life. Manufacturing of tablets includes a number of processes through which a tablet is formed. The chemicals and other aspects that are being used to form a tablet can be harmful for humans if there is any mistake while the tablet is being manufactured. Some factors have been identified that are responsible for the negative impacts of tablets such as:
Poor tablet design: design of the tablet matters a lot in the overall presentation of the tablet. Sometimes the surface of the tablet is thick and the wall of the tablets seems to be over straight with thick edges (Ketterhagen et al., 2017). These are not the right shape for a tablet as these can break the entire positivity of the tablet. Sometimes the fillers that are being contained by the tablet made by minerals such as carbonate or calcium sulphate are extremely harmful for the human body. The suspension of the tablet design needs to be reformulated in terms of improving the tablet design which can cause a fine embossing of the tablet. It is another issue that can be raised in the formation of a tablet which is equally harmful for human body.
Physical damage: Wrong or improper design of the tablet can cause to the physical damage of the tablet. The coating element that is being used to coat the tablet should be in a limited percentage. The overloading of the coating element can make a physical damage in the tablet as well as the human body (Murillo et al., 2019). Sometimes, the used elements are not sustainable for the human body as they cannot be dissolved in the human body and cause another difficulty. It is also noted here in risk management plan assignment that the operations that are aligned with the manufacturing process of the tablet must be in the limit that it cannot be able to lead the positive vibes of the tablets towards negativity. Physical damage of the tablets can cause a huge disaster as sometimes the opacity of the tablet is destroyed which is not good for the human body. The improper surface of the tablet can also be the cause behind the physical damage of the tablet.
Over wetting: the surface of the tablet gets hit by the droplets of the suspension area and the coating area of the tablet gets wet (Murillo et al., 2019). The drying air that is used to dry the air in this situation does not help the situation and the wet portion cannot be dried up easily. For this issue, the entire coating form of the tablet remains wet and the structure of the tablet starts to break inside. Sometimes it causes a serious damage which is not visible from outside. Surface pitting is the most effective negative impact of this issue. According to the research on the case of risk management plan assignment, the broken structure of the tablet can harm a human body after intake as the coating area of the tablet cannot be dissolved while it is wet.
Spray drying: the coating area of the tablet get hit by the droplets of the suspension area just after the removement of the moisture that has been used in the manufacturing process (Mohammed, 2016). A lack of proper adherence has been noticed in this situation as this issue leads towards the poor coating. The coating of the tablet plays an immersive role in the allover formation of the tablets. Sometimes the gun position is not right that is used in the manufacturing process of the tablet. The distance between the gun and the tablet bed is not maintained sometimes which causes damage in spray drying of the tablet (Murillo et al., 2019).
What is the role of risk assessment in this segment of risk management plan assignment?
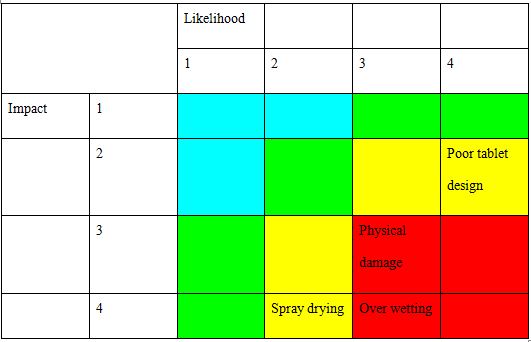
The concept of the risk assessment deals with the usability and adherence of influenced and effective work function (Dennison et al., 2016). The assessment of the risk would be helpful for the identification of the conceptual and effective management of smart documentation. The alignment of the risk assessment would help in listing the impacts of the risk and aligning the continuation of the improved mitigation. The context of risk management plan assignment also signifies the use of the proper and formal risk alignment would be helpful for aligning the continuation of the works. The risk assessment would be helpful for the successful and effective identification of the factors of the risk and their conceptual deployment of the operations. The identified risks of poor tablet design, physical damage, over wetting, and spray drying. These have been assessed for the likelihood and the impact identification of the works. The following is the risk assessment for the pharmaceutical product tablet,
|
Risk |
Description |
Likelihood |
Impact |
Rating |
|
Poor tablet design |
Now and then the outside of the tablet is thick and the mass of the tablets is by all accounts over straight with thick edges. These are not the correct shape for a tablet as these can break the whole inspiration of the tablet. |
4 |
2 |
8 |
|
Physical damage |
The over-burdening of the covering component can make a physical harm in the tablet just as the human body (Haleem et al., 2015). Now and then, the utilized components are not supportable for the human body as they can not be broken up in the human body and cause another trouble. Physical harm of the tablets can cause a gigantic debacle as in some cases the murkiness of the tablet is annihilated which isn't useful for the human body. |
3 |
3 |
9 |
|
Over wetting |
The drying air that is utilized to dry the air in this circumstance doesn't support the circumstance and the wet segment can not be evaporated effectively. Surface setting is the best negative effect of this issue. |
3 |
4 |
12 |
|
Spray drying |
The covering region of the tablet get hit by the beads of the suspension region soon after the removement of the dampness that has been utilized in the assembling procedure (Su et al., 2018). The covering of the tablet assumes a vivid job in the allover arrangement of the tablets. |
2 |
4 |
8 |
Risk Priority Matrix
The risk priority matrix explored in this case of risk management plan assignment is helpful for identifying the effective and impactful alignment of the risk and classifying them based on their likelihood and impact (Almeida, Hankins & Williams, 2017). The assessment of the risks of pharmaceutical product tablet would help in identifying the priority of the risk for identifying the respective management of the works. The risk priority matrix based on the case of risk management plan assignment is given below,
Risk Mitigation
The following report on risk management plan assignment is based on the identification of the risk factors in the formation of the tablets through the implementation of a strong risk management plan. Some of the mitigations utilized in the present case of risk management plan assignment are mentioned below:
Prescribed training: the employees who are working on the manufacturing process of the tablets must be trained properly. The members should be aware of what can be the consequences of using the coating elements more than the essentiality (Hopkin, 2018). Along with that, how to make the tablet surface perfect or how to do spray drying aligned with the usage of coating color should be enlisted in their knowledge.
Monitoring strategy: there must be a project manager who would keep an eye on the activities of the project to monitor. What coating element is being used in what proportion must be monitored by the in-charge to avoid all the unintended obstructions (American Diabetes Association, 2016). Entire process should be in control that the negative impacts can be reduced.
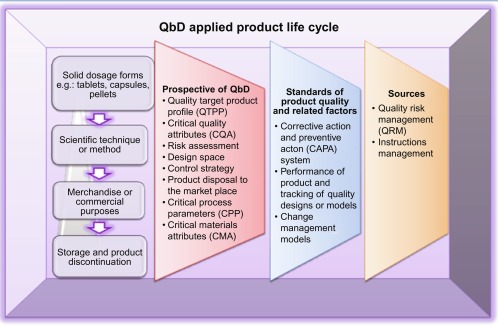
Figure 1: Role of Risk Quality Management in Medical Tablets Development Steps
(Source: Gilani et al., 2019)
Awareness programs: the authority can think about raising some awareness programs that the other community can have some basic knowledge. It would help to have some precautions to avoid harmful impacts of the improper coating.
Conclusion
It can be concluded after analyzing the above context of risk management plan assignment that the assessment of the risk for the inclusion of the facilities can take care of the operations. The assignment had taken care of the factors that can support the effective management of the operations. The usability of the factors can also take care of the explicit management of the successful development of the mitigation strategies for the identified issues. The risk factors for the pharmaceutical product tablet are poor tablet design, physical damage, over wetting, and spray drying. The effective management of the operations and developed for the conceptualization of the working development. The present scenario of risk management plan assignment also elucidates that the priority matrix would be developed for prioritizing the successful and effective implication of the work. The enabling of the facilities can enable the usage of influenced management process. The inclusion of the effective documents that can align with the concentrated and managed risk assessment would be supportive for alignment.
References
Almeida, H., Hankins, K. W., & Williams, R. (2017). Risk management plan assignment Risk management with supply contracts. The Review of Financial Studies, 30(12), 4179-4215.
American Diabetes Association. (2016). 8. Cardiovascular disease and risk management. Diabetes care, 39(Supplement 1), S60-S71.
Dennison, T. J., Smith, J., Hofmann, M. P., Bland, C. E., Badhan, R. K., Al-Khattawi, A., & Mohammed, A. R. (2016). Design of experiments to study the impact of process parameters on droplet size and development of non-invasive imaging techniques in tablet coating. PloS one, 11(8), e0157267.
Gilani, S. J., Rizwanullah, M., Imam, S. S., Pandit, J., Aqil, M., Alam, M., & Beg, S. (2019). QbD Considerations for Topical and Transdermal Product Development. In Pharmaceutical Quality by Design (pp. 131-150). Academic Press.
Haleem, R. M., Salem, M. Y., Fatahallah, F. A., & Abdelfattah, L. E. (2015). Quality in the pharmaceutical industry–A literature review. Saudi Pharmaceutical Journal, 23(5), 463-469.
Hopkin, P. (2018). Fundamentals of risk management: understanding, evaluating and implementing effective risk management. Kogan Page Publishers.
Ketterhagen, W., Aliseda, A., am Ende, M., Berchielli, A., Doshi, P., Freireich, B., & Prpich, A. (2017). Modeling tablet film-coating processes. In Predictive Modeling of Pharmaceutical Unit Operations (pp. 273-316). Woodhead Publishing.
Lin, H., Dong, Y., Markl, D., Zhang, Z., Shen, Y., & Zeitler, J. A. (2017). Pharmaceutical film coating catalog for spectral domain optical coherence tomography. Risk management plan assignment Journal of pharmaceutical sciences, 106(10), 3171-3176.
Murillo, M. A., Rodríguez?Pulido, F. J., Heredia, F. J., Melgosa, M., Pacheco, J., Vargas, R., ... & Gutiérrez, D. (2019). Color evolution during a coating process of pharmaceutical tablet cores by random spraying. Color Research & Application, 44(2), 160-167.
Su, Q., Moreno, M., Ganesh, S., Reklaitis, G. V., & Nagy, Z. K. (2018). Resilience and risk analysis of fault-tolerant process control design in continuous pharmaceutical manufacturing. Risk management plan assignment Journal of Loss Prevention in the Process Industries, 55, 411-422.