Artificial Intelligence Assignment: A SystematicReview of Artificial Intelligence in Oncological Imaging
Question
Task: You are required to prepare a detailed and well-researched report on artificial intelligence assignment on the topic “A systematic review of Artificial Intelligence in Oncological imagingwithin the context of Prostate cancer diagnosis and management.”
Answer
Abstract
The artificial intelligence assignment is focused on the management and diagnosis of prostate cancer in terms of oncological imaging which integrates the interpretation of data from different modalities to assist in informed and effective decision-making. In addition, artificial intelligence commits to make great advances in the qualitative interpretation of cancer imaging by clinicians including treatment on adjacent organs, volumetric delineation of tumors over time, assessment of the impact of the disease, extrapolation of the tumor genotype, prediction of clinical outcome as well as the biological course from radiographic phenotype. Artificial intelligence can automate the processes in the preliminary detections, administering an intervention, management decisions as well as subsequent observation. Other than that, AI can assist clinicians to comprehend complex relationships and manage enormous data sets which are highly time-consuming and difficult for humans. This means by integrating AI algorithms as well as decreasing the level of subjectivity, it is feasible to use lesser resources while enhancing the accuracy and efficiency in prostate cancer diagnosis and management within the context of oncological imaging.
Here, an in-depth and systemic approach has been integrated to review the current state of artificial intelligence as applied to oncological imaging with an explicit focus on prostate cancer to highlight how this clinical problem is being addressed. On the contrary, it has been analysed that most of the research studies analysing artificial intelligence applications in oncology have not been substantially validated for generalizability and reproducibility but the results and discussions significantly illustrate rising concerted efforts in reinforcing artificial intelligence technology to clinical use specifically for oncological imaging for impacting future directions in cancer care with a significant focus on prostate cancer.
Introduction
Prostate cancer is one of the most common diagnosed non-skin malignancies in men as well as the second major reason for mortality from cancer. In addition, it is projected that 1 in 6 American men could be affected by prostate cancer during their lifetimes [6]. In the pre-PSA era, it has been analysed those patients with prostate cancer generally presented with symptoms that encompassed hematuria, urinary complaints or retention as well as back pain. The PSA is significantly used for the diagnosis and management of prostate cancer as it is the unique serum biomarker. On the contrary, the major challenges faced by clinicians include a higher volume of prostate biopsies as well as the shortage of urological pathologies that directly increases pressure on the diagnosis of prostate cancers. Other than that, the presence of variability in terms of pathological grading can lead to under-treatment or over-treatment of prostate cancer. At present, with PSA screening and imaging, most prostate cancers are diagnosed early at an asymptomatic stage. However, when PSA is abstemiously increased ranging from 4-10 ng/ml, it becomes highly complex and challenging for clinicians to distinguish cancer from benign prostatic hyperplasia [2].
Likewise, AI and ML in healthcare have been a potential and new area of research that has certainly gained multiple interests [3]. The ANN uses a statistical model that is influenced and modeled by the biological neural networks which are often used by AI. Their ability to model and process non-linear relationships between outputs and inputs in parallel has been highly effective and efficient. In oncology, imaging is a subspeciality that is highly focused on the treatment as well as diagnosis of cancer. In addition, by using strong imaging modalities such as PET/CT, CT, MRA, MRI, clinicians are able to assist in the detection, diagnosis, and management of cancer. This is where the potential significance of AI can be utilized [4]. In prostate cancer, the use of AI has been beneficial to support standardised pathological grading to analyse prostate cancer treatment and stratification. Other than that, AI integrates automation in the assessment of severity and characterization of prostate cancer in terms of imaging-based tasks such as biomarker diagnosis, histopathologic, and MRI. Likewise, patients that are diagnosed with prostate cancer can continue with repeated surveillance and imaging through PSA, prostate biopsies as well as other forms of digital testing such as rectal examinations or MRI while considering physiological side effects.
It is expected that the field of urology and urological pathologists can enhancetheir diagnosis, prognosis,and treatment of patients by capitalizingon the potential benefits of AI in the significant forms of surveillance and is projected to become an essential tool as a whole. Based on such context, the purpose of this systematic review is to determine whether artificial intelligence can be applied to distinguish GS 7 (4+3) or more from GS 7 (3+4) or less, in terms of combined radiological mpMRI as well as digital pathology data for enhanced prognostication of prostate cancer and better clinical care. In addition, the review of recent advances in the context of AI and ML in oncological imaging with particularfocus on the prostate can diagnosis and management has been further discussed.
Materials and methods
It has been well indicated that a systematic review can be defined as a review of the evidence on explicit formulate questions that utilize both explicit and systematic methods to determine, select as well as assist to extract and analyse data from the studies are integrated into the review [15].The present study has considered the use of PubMed, Google Scholar, and Medline databases for developing a systematic review of the literature in order to assess the significance and application of AI in oncological imaging with regards to prostate cancer. The review has been performed in terms of the Preferred Reporting Items for Systematic Review and Meta-analysis. The keywords used include (artificial intelligence) or (deep learning) or (prostate cancer) or (clinicaloncology) or (machine learning) or (prostate imaging) or (artificial neural network)or (prostate biopsy) and (clinical trial). Based on such context, preliminary results were gathered that indicated around 220 manuscripts. Likewise, searching multiple databases can result in both published and unpublished literature based on which credible and valid sources have to be selected in order to accomplish generalized results and outcomes [11].Based on such context, an exclusion criterion was adopted for removing duplicate manuscripts which have resulted in 165 manuscripts. Furthermore, other exclusion criteria were included: animal studies, case reports, surveys, review, commentary, opinion or blogs, and also the manuscripts that were not specific to the particular topic in question. This narrowed down into 42 full-text manuscripts in terms of eligibility, credibility, and validity. After conducting a full-text assessment in order to integrate credible sources and valid results, 12 valid and credible manuscripts have been considered in this systematic review.
Results
Several studies have determined the utility of PSA as well as other clinical characteristics for detecting prostate cancer as well as its progression in terms of the setting of an ANN. Based on such context, an ANN has been developed to improve the specificity and sensitivity of prostate cancer detection [7]. The study integrated 1246 participants (men) with the major aim to predict the presence of benign prostatic tissue or prostate cancer tissue with serum total PSA levels from 4-10 ng/mL and 2.5- 4 ng/mL which has been compared to conventional tools. The result indicated that 95% sensitivity. The ANN model developed for patients with total PSA levels from 4-10 ng/mL highlighted receiver operator characteristics curve area, specificity, negative as well as the positive predictive value that were primary to other comparativeparameters.Likewise, based on the above study, [10] also developed two significant models including the ANN model and logistic regression model to support the diagnosis and management of prostate cancer. In order to derive prostate imaging, the predictors including PSA density, age, prostate volume, and percent free PSA from 586 respondents (men) to train their algorithms in terms of prostate cancer as confirmed by biopsy. Also, [17] indicated that automatic classification by utilizing ML can offer more consistent and accurate results for aiding management and decision support for clinicians by combining T2-weighted MRI-based texture features and apparent diffusion coefficient through ML in terms of prostate cancer surveillance and imaging. The CNN model that integrates DL toll can assist to look at recurrence in conjunction to indicate the scores for accuracy and imaging.
On the other hand, it has been well argued by [8] that using free PSA integrated with an ANN with the aim to eliminate unnecessary screening and imaging for prognosis of prostate cancer. The model has been developed using total PSA data ranging 4- 10 ng/mL, digital rectal examination, an appropriate proportion of free PSA, and prostate volume from 656 participants (men). The results indicated that 19% of falsepositives can be avoided by using the appropriateproportion of free PSA (33% with the ANN model and 24% with the logistic regression model) versus at a 95% sensitivity level. Hence, these findings are highly valuable and accurate in oncological imaging specifically in the context of prostate cancer. As stated by [16], an AI system such as trained ANNs can assist to evaluate access to prostate biopsies as well as offer a standardised grading method which is essential for pathology expertise in underserved areas. The study used digitized slides from 1247 participants (men) and trained ANNs for the purpose. The networks were assessed by predicting Gleason grade, presence, and extent of malignant tissue. As a result, the AI tool accomplished an area under the ROC curve of 0.997 to differentiate malignant and benign scores of biopsies, (Refer to the below figure). In addition, [18] further investigated the prostate cancer detectionanddetermination of aggressiveness in terms of MRI that indicated the need for AI and ML to distinguish high and low Gleason grades.
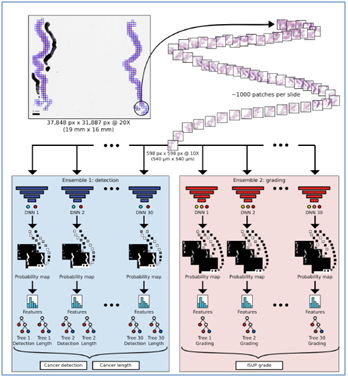
Fig: 1 (Overview of the artificial intelligence system in terms of oncological imaging)
Source: (Strom et al. [16])
A study to compare the diagnostic performance of a standard six-pulse sequence multiparametric MR imaging protocol versus a short dual-pulse sequence magnetic resonance (MR) imaging protocolwas conducted to detect clinically significant prostate cancer [1]. The study integrated 63 patients (men) between 2013-2015 based on significant characteristics who underwent MR imaging of the prostate including consideration of other variables and modalities where Trans-perineal template saturation biopsy was considered as the standard of reference.The results revealed that thereis no difference in detection of tumors bigger than or equal to 0.5 mL and 84 quadrats were positive for cancer in 28 patients out of 63.In addition, [5] along with [16] further suggested that the AI methods can assist to addresses the problems and challenges including over or under-treatment of prostate cancer and its grading system by assisting pathologists and clinicians to focus on harmonizing grading and reducing workload at an extensive level, (Refer to the below fig: 2). In order to assess the performance of the AI for detection and grading cancer in the prostate, 976 participants were considered and STHLM3 diagnostic study was considered to tarin deep neural networks for evaluating prostate biopsies. The result of the study indicated that AI achieved an are in termsof the ROC curve and distinguished cancer areas effectively in terms of Gleason grades as well.
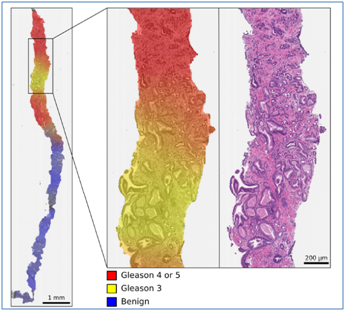
Fig: 2 (Effective AI integration for estimating cancer grades in terms of color-coded visualization)
Source: (Strom et al. [5])
Pathologic grading plays a significant role in the prostate can risk treatment selection as well as stratification. Likewise, a novel approach to combine genomic with AI along with cell-free DNA can offer consistent and rapid tests of PCa[12].In addition, mpMRI has further become a well-established tool for localizing and detecting prostate cancer. On the contrary, both radiologic and pathological assessments suffer from poor reproducibility. Hence, the integration of AI in the detection of assessment of imaging-based tasks basedon high-quality training sects can be well applied to mpMRI as well as digital pathology in prostate cancer. On the other hand, it has been further indicated that artificial intelligence when coupled with radiomics can assist to handle a massive amount of data meaning the extraction of a high number ofquantitative data from medical images which can be used for decision support [14]. As fear of replacement by intelligent machines has been significant among radiologists, the need to become familiar with AI and computational power has to be well considered in future clinical practice. Other than that, deep learning and machine learning has the potential to address existing technological challenges of traditional imaging-based tasks by introducing mpMRI images of the prostate and better segmentation, texture analysis, and extraction of data through advanced AI integration.
ANNs can play a significant role in the analysis and validation of the biomarkers. Based on such context, [9] indicated that it is essential to identify and evaluate biomarkers that can be used for clinical signs such as Ki67, an essential marker of survival as well as disease progression. In order to confirm this, they developed an ANN that was designed to analyse and validate Ki67 gene expression by comparing it to another potential variable in DLX2. By adopting univariate analysis, it was concluded that both DLX2 and Ki67 were important in the predictiveness of metastases in the future. Other than that, around 6.8% of patients with prostate cancer reflected high expression of Ki67. Hence, this study put forward that both DLX2 and Ki67 biomarkers can be utilized to assess patients or respondents for a targeted therapy only. On the other hand, proteomics is also useful when identifying significant biomarkers in addition to gene expression. A novel approach was developed to combine targeted proteomics with computational biology for discovering new potential signatures of proteomic for prostate cancer [13]. By integrating synthetic peptides among the 74-patient cohort while evaluating 133 different proteins, they were applied in the machine learning approach in order to create clinical predictive models. The results further indicated that computationally guided proteomics can be utilized to identify and validate novel non-invasive biomarkers. Hence, it can be stated that there are several studies that highlight the potential of AI and ANNs to enable the effectiveidentification and validation of biomarkers for complementing prostate cancer surveillance.
Discussion
The AI type ANN + PSA dx is a significant tool for statistical prediction of early prostate cancer that uses PSA levels. In addition, the ANN accuracy and specificity level has been higher compared to other conventional multivariate analyses and PSA parameters. It can be further stated that the model has high diagnostic validity as well as the potential to be included in practice for avoiding unnecessary biopsies and offering quality care and treatment to patients suffering from prostate cancer. Hence, this AI type has the potential for predicting early prostate cancer by oncological imaging. Other than that, the including ANN model and logistic regression model does not have significant difference but the models have significant value into prostate cancer screening to prevent false-positive results as well as can identify men at increased risk of a positive prostate biopsy for cancer. Therefore, it can be stated that by combining multiple studies, the significance and relevance of using AI-based ANN tools can be effective and efficient in the detection and imaging of prostate cancer at an early stage.
On the other hand, the ANN + PSA dx has been also developed in terms of multivariate algorithms with regards to clinical characteristics for decreasing the rate of false-positive in prostate imaging and screening which integrates a higher level of free PSA. Furthermore, the logistic regression model highlighted higher sensitivity and accuracy when compared to free PSA indicating a positive attributed to a decrease in the number of unnecessary prostate biopsies. Likewise, the studies further indicate that the capacity of ANNs to evaluate prostate biopsies in terms of Gleason grade, presence, and extent of the malignant tissue with regards to experienced urological pathologists is immense. It also indicated that an ANN is highly capable to be trained for detecting and assessing prostate biopsies providing similar accuracy and specificity in terms of urological pathologists. This means that the integration of AI in oncological imaging specifically in thecontext of prostate concern is significant and is highly essential for the purpose of urological pathologists in its treatment, diagnosis, and management. In addition, the ability ofpathologists or clinicians to distinguish benign and malignant tissue can be highly advanced by the use of AI and ANNs.
In addition, utilizing machine learning-based automatic classification of prostate cancer while combining T2-weighted MRI-based texture features and apparent diffusion coefficient can assist to differentiate between 7 (3+4) and 7 (4+3) cancers. This model can assist to predict cancers through accurate imaging in both transition and peripheral zones of the prostate with 93% accuracy. Additionally, MRI and its combination with T2-weighted MR imaging and apparent diffusion coefficient can be well benefited by integrating AI algorithms for easy detection of malignanttumors and offer reliable solutions.It has been further comprehended that for the early detection of clinically explicit prostate cancer, there were no significant differences in the diagnostic performance of the short MR imaging protocol that encompasses traverse T2-weighted as well as diffusion-weighted imaging pulse sequences while comparing it to the standard multiparametric MR imaging protocol.
Other than that, the application of AI and machine learning tools can assist to estimate the probability of high-grade malignancy, benign tissue as well as low-grade malignancy at different areas of the specimen with regards to the output that corresponds to histological images. Furthermore, the ANN + biomarker dx model has been developed as the predictive biomarkers for the result in DLX2 and KI67. The outcomes further highlighted being able to inform effective decision-making in terms of clinical practice within patients based on active surveillance. It also suggests that Ki67 is an essential biomarker of survival as well as disease progression where ANNs play a significant role in validating and analysing the biomarkers.As there are several biomarkers and no justified standard, there is not a perfect list of biomarkers that can predict diagnosis or prognosis. Hence, it is essential to identify and evaluate new biomarkers like Ki67 that are meaningful and accurate in terms of clinical significance which has been potentially identified in the studies.
On the other hand, it has been further comprehended that targeted proteomics can be used to discover potential proteomic signatures for oncological imaging in terms of prostate cancer. In addition, computationally guided proteomics can be further utilized to identify and validate accurate non-invasive biomarkers with regards to ANN + biomarker dx AI type. As it has been established in the past studies that mpMRI suffers from poor reproducibility in terms of radiologic and pathologicalassessment for detecting and localizing prostate cancer. This means that the application of AI to digital pathology and mpMRI can allow advanced characterization of disease in terms of prostate cancer and imaging-based tasks by combining with radiology-pathology assessment.Furthermore, AI integrates radiomics and textural analysis that assist to distinguish between high-grade and low-grade tumors irrespective of the need for gene expression-based molecular signatures.One of the key advantages of combining AI and radiomic is it can assist to predict PCa’s grade groups which can be used to develop strategy, course of treatment, and management of prostate cancer.
Conclusion
In conclusion, it can be stated that the use of artificial intelligence for managing medical issues and challenges has been an interesting area for quite some time that entails potential discussions and arguments. However, the recent advancement in technologies hasassisted clinicians and pathologists to make significant growth. AI can enable to recognitionof complex relationships as well as managinghuge data sets which is a complex and time-consuming task for humans. In addition, AI has the capability to diagnose and stratify risks that is needed for active surveillance trials. By reducing the level of subjectivity and using AI algorithms, it is possible to utilize lesser resources and to enhance the overall accuracy and efficiency in trials in terms of oncology imaging for detecting and analysing cancerous tissue. As the economic burden of the management of localised prostate cancer required significantacknowledgement, the need to manage both low and high-risk patients suffering from prostate cancer has been estimated to cost extremely.
On the contrary, such challenges can be well addressed with the help of AI whilemaintaining and enhancing current outcomes in active surveillance trials. By the application of AI in terms of computational algorithms including DL and ML, the diagnosis, prognosis as well as imaging of prostate cancer can be made at ease with effective cost reduction aiding clinical practice. Other than that, biomarker data well as radiomics can be well integrated with AI to support decisions and for enhanced oncologic management decisions.Hence, it is significant to acknowledge and enhance the current pipelines to drive the future, reduce costs and enhance outcomes of urologic oncology with a specific focus on prostate cancer and imaging-based tasks.
References
1. Barth BK, De Visschere PJ, Cornelius A, Nicolau C, Vargas HA, Eberli D, Donati OF. Detection of clinically significant prostate cancer: short dual–pulse sequence versus standard multiparametric MR imaging—a multireader study. Radiology. 2017 Sep;284(3):725-36.
2. Berlin A, Castro-Mesta JF, Rodriguez-Romo L, Hernandez-Barajas D, González-Guerrero JF, Rodríguez-Fernández IA, et al. Prognostic role of Ki-67 score in localized prostate cancer: A systematic review and meta-analysis. UrolOncolSeminOrigInvestig. (2017) 35:499–506.
3. Berney DM, Gopalan A, Kudahetti S, Fisher G, Ambroisine L, Foster CS, et al. Ki-67 and outcome in clinically localised prostate cancer: analysis of conservatively treated prostate cancer patients from the
4. Bresnick J. Artificial Intelligence in Healthcare Spending to Hit $36B.(2018).
5. Ström P, Kartasalo K, Olsson H, Solorzano L, Delahunt B, Berney DM, Bostwick DG, Evans AJ, Grignon DJ, Humphrey PA, Iczkowski KA. Pathologist-level grading of prostate biopsies with artificial intelligence. arXiv preprint arXiv:1907.01368. 2019 Jul 2.
6. Chaddad, A.; Kucharczyk,M.J.; Cheddad, A.; Clarke, S.E.;Hassan, L.; Ding, S.; Rathore, S.;Zhang, M.; Katib, Y.; Bahoric, B.; et al. Magnetic Resonance Imaging Based Radiomic Models of Prostate Cancer: A Narrative Review. Cancers 2021, 13, 552.
7. Djavan B, Remzi M, Zlotta A, Seitz C, Snow P, Marberger M. Novel artificial neural network for early detection of prostate cancer. Journal of Clinical Oncology. 2002 Feb 15;20(4):921-9. doi:10.1200/JCO.2002.20.4.9219
8. Finne P, Finne R, Auvinen A, Juusela H, Aro J, Määttänen L, Hakama M, Rannikko S, Tammela TL, Stenman UH. Predicting the outcome of prostate biopsy in screen-positive men by a multilayer perceptron network. Urology. 2000 Sep 1;56(3):418-22. doi:10.1016/S0090-4295(00) 00672-5
9. Green WJ, Ball G, Hulman G, Johnson C, Van Schalwyk G, Ratan HL, Soria D, Garibaldi JM, Parkinson R, Hulman J, Rees R. KI67 and DLX2 predict increased risk of metastasis formation in prostate cancer–a targeted molecular approach. Artificial intelligence assignment British journal of cancer. 2016 Jul;115(2):236-42.
10. Ge P, Gao F, Chen G. Predictive models for prostate cancer based on logistic regression and artificial neural network. In2015 IEEE International Conference on Mechatronics and Automation (ICMA) 2015 Aug 2 (pp. 1472-1477). IEEE.
11. Granja C, Janssen W, Johansen MA. Factors determining the success and failure of eHealth interventions: systematic review of the literature. Journal of medical Internet research. 2018 May 1;20(5):e10235.
12. Harmon SA, Tuncer S, Sanford T, Choyke PL, Türkbey B. Artificial intelligence at the intersection of pathology and radiology in prostate cancer. Diagnostic and Interventional Radiology. 2019 May;25(3):183. doi: 10.5152/dir.2019.19125
13. Kim Y, Jeon J, Mejia S, Yao CQ, Ignatchenko V, Nyalwidhe JO, Gramolini AO, Lance RS, Troyer DA, Drake RR, Boutros PC. Targeted proteomics identifies liquid-biopsy signatures for extracapsular prostate cancer. Nature communications. 2016 Jun 28;7(1):1-0.
14. Koçak B, Durmaz E, Ate E, Klçkesmez Ö. Radiomics with artificial intelligence: a practical guide for beginners. Diagnostic and Interventional Radiology. 2019 Nov;25(6):485.
15. Pati D, Lorusso LN. How to write a systematic review of the literature. HERD: Health Environments Research & Design Journal. 2018 Jan;11(1):15-30.
16. Strom P, Kartasalo K, Olsson H, et al. Artificial intelligence for diagnosis and grading of prostate cancer in biopsies: a population-based. Diagnostic Study, Lancet Oncol. 2020; 21:222–232. doi:10.1016/S1470-2045(19)30738-7.
17. Suarez-Ibarrola R, Hein S, Reis G, Gratzke C, Miernik A. Current and future applications of machine and deep learning in urology: a review of the literature on urolithiasis, renal cell carcinoma, and bladder and prostate cancer. World journal of urology. 2020 Oct;38(10):2329-47.
18. Zapatero A, Guerrero A, Maldonado X, Alvarez A, San Segundo CG, Rodríguez MA, Macias V, Olive AP, Casas F, Boladeras A, de Vidales CM. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. The Lancet Oncology. 2015 Mar 1;16(3):320-7.