Biology Assignment: Role Of Aldehyde Dehydrogenase For Treating Cancer
Question
Task:
You are supposed to prepare a biology assignment on the topic “High levels of aldehyde dehydrogenase (ALDH) leads to chemo- and radioresistance, but can their functional activity also be exploited for patient benefit?”.
Answer
Introduction
This biology assignment critically examines aldehyde dehydrogenase which is a subfamily of phase-I oxidases, accountable for maintaining cell homeostasis, via assimilating both exogenous and endogenous reactive compounds. Aldehyde dehydrogenases are a set of enzymes able to stimulate aldehyde oxidation. These enzymes are able to convert aldehydes to carboxylic acids. In human genome, there are 19 ALDH genes, which give rise to 19 different types of ALDHs (Ma & Allan, 2010). The products of these genes are participating in different types of physiological processes, such as detoxification of exogenous and endogenous aldehydes, detoxification of alcohol-derived acetaldehyde, development of vital molecules, including retinoic acid, which is crucial for eye functioning. This polymorphic enzyme stimulates aldehydes oxidation to carboxylic acids, leaving liver and being metabolized by heart and muscle tissue. The recent research has highlighted the role of this enzyme in cancer therapy. Some evidences suggested that over expression of these proteins lead to resistance to conventional cancer therapy, but its increasing research is investigating its functional role for using its benefits in cancer therapy. The following review is going to critically appraise existing literatures for exploiting opportunities and obstacles related to its role in beneficial cancer treatment.
Discussion
Aldehyde dehydrogenases (ALDHs)
As per the recent research, three different categories of ALDHs have been identified, i.e. “class 1 (low Km, cytosolic), class 2 (low Km, mitochondrial) and class 3 (high Km, such as those expressed in tumors, stomach, and cornea)”. All of these enzymes exist in two forms, i.e. constitutive and inducible form. The mostly known and important types include ALDH1 and ALDH2 involved in aldehyde oxidation. Both of these enzymes are tetrameric and consists four 54 kDa subunits. Usually, these enzymes are found in different body tissues, but maximum abundance is seen in liver. Januchowski, Wojtowicz and Zabel (2013) highlighted that the enzyme is highly conserved through its different categories. Among these categories, the number of amino acids in each subunit can be changed, but on the whole, role of the site usually remains intact. The active side of the enzyme binds with one molecule of an aldehyde and another one is with NAD+ or NADP+, which is used in the reaction as a cofactor. The key reaction catalyzed by ALDH enzyme is as follows:
RCHO + NAD+ + H2O ? RCOOH + NADH + H+
Functional role of Aldehyde dehydrogenases (ALDHs) in normal and progenitor cells
Aldehyde dehydrogenases are crucial for maintenance as well as differentiation of stem cells. These enzymes play key role in their development too. Several studies have highlighted the function of ALDH1A1 in stem cells and it is increased in hematopoietic stem cells (HSCs). In this regards, Gasparetto and Smith (2017) attempted to better characterize these cells for which they developed fluorescent ALDH1A1 substrate Aldefluor for promoting identification and isolation of HSC. The researchers found it important for continuing and isolating HSCs from different tissues, i.e. peripheral blood, human bone marrow and cord blood, along with stem cells in other organisms and tissues. Considering the elevated level of expression of ALDH1A1, the researchers investigated the biology behind it, along with other ALDH categories in HSCs (Xu et al., 2015). It is revealed that ALDH1A1 and ALDH3A1 are crucial for assimilating reactive aldehydes and reactive oxygen species. They studies in murine models and revealed that losing these isoforms led to different effects in HSC, like enhanced predisposition to leukemia development, DNA damage, upon combining with a genetic stimulator of HSC proliferation and self-renewal. Losing ALDH activity has been seen to predispose to marrow failure and “AML in Fanconi’s anemia”.
Pors and Moreb (2014) found that human being hematopoietic progenitor cells develop and impediment differentiation in vitro, after being treated with chemical ALDH1 inhibitor, i.e. “diethylaminobenzaldehyde (DEAB)”. Overexpression of ALDH1A1 in hematopoietic cells gives resistance to cyclophosphamide. Therefore, ALDH1A1 could be an important regulator of stem cell task and the key determinant of ALDH activity through Aldefluor assay in usual tissue stem cells.
Different studies have found that ALDH1 super family is highly active in common tissue stem cells, which makes it to be deemed as a marker for stem cells. It is playing a fundamental role in differentiation, expansion and self-protection of stem cells. Additionally different isoforms of ALDH, including “ALDH1A1, ALDH1A2, ALDH1A3, and ALDH8A1 are playing significant role in RA formation through oxidation of all-trans-retinal and 9-cis-retinal being involved in retinoid signaling. These isoforms are also associated with stemness of stemcells (Sládek et al., 2002).
Functional role of Aldehyde dehydrogenases (ALDHs) in cancer stem cells
Normal tissue stem cells and cancer stem cells usually share different biological mechanisms, but there are some differences too. Currently, there are debate related to differences within normal tissue stem cells and cancer stem cells. It is yet not clear that unlike the normal tissue stem cells, which are oligo or multipotent, whether cancer stem cells are capable to produce to multiple differentiated cell types. Further, it is also not clear whether normal cellular precursor of cancer stem cells are true normal stem cells or not (Muralikrishnan, Hurley & Nephew, 2020). For instance, in case or normal stem cells, the surrounding microenvironment is normal, but in case of cancer stem cell, they possess aberrant microenvironment, including inflammation, low nutrient conditions and hypoxia.
The practical role of ALDH1A1 has been described in that cells with high ALDH1 from breast cancer identified by Aldeflour assay were found to be tumor-initiating cells. It indicates that ALDH1A1 has potential for driving tumor proliferation, maintenance and differentiation. This further influenced researchers to think about differentiation and maturation of cancer stem cells to be cancer therapy for objecting these cells (Januchowski, Wojtowicz & Zabel, 2013).
Alison et al., (2010) claimed that predictive data on ALDH1 in different cancers have been gathered with the use of IHC of paraffin implanted cancer tissue with isotyoe-specific antibodies, i.e. ALDH1A1 or ALDH1A3. Increased expression of ALDH1A1 has been assessed by IHC considering correlation with poor and supportive prognosis in different types of cancers. In contrast, experimental researches of ALDH activity of cancer stem cells showed that ALDHbri cells alienated by Aldeflour assay are having more tumorigenic potential both in vitro and in vivo conditions. Overall, researchers revealed that enhanced expression of ALDH is associated with worsened prognosis with very few exemptions, like melanoma. Additionally, IHC analysis of ALDH1A1 expression with isotype-specific antibody, like ALDH1A1 antibody, highlighted favorable prognosis of different cancer types (Tomita et al., 2016). The researchers claimed that such differences might be linked with differentiation and maturation of ALDH1A1 positive cells in cancer types. Critical review of existing studies revealed significant discrepancies among results (Raha et al., 2014). The reason behind this issue could be the detection method for ALDH, type of tissue handling, cut-off level of ALDH1A1 staining as well as histological type of studies. Further, high activity of ALDH1 by Aldeflour assay is associated with worsened prognosis. It is because; this assay identifies different ALDH1 isoforms. In recent studies, it has been highlighted that ALDH1A3 is contributing to Aldeflour high activity, which could be cancer type and tissue specific, like human breast cancer stem cells and in murine hematopoietic stem cells, murine pancreatic progenitor cells (Moreb, Ucar-Bilyeu & Khan, 2017). Therefore, one must be cautious in evaluating over-expression and elevated activity of ALDH1A1 in different types of cancer.
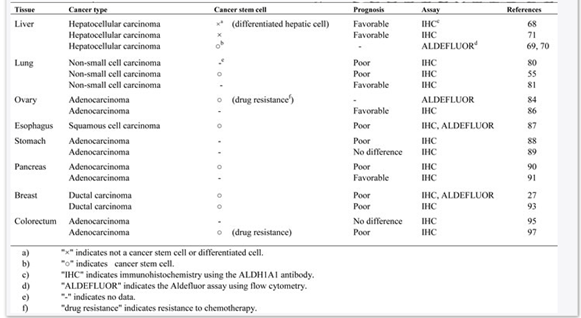
Table: ALADH1A1 overexpression in different cancer stem cells and cancer cell types
(Source: Tomita et al., 2016)
Aldehyde dehydrogenases (ALDHs) as a marker for normal stem cells
Previous studies identified that ALDHbr family are highly enriches I lineage-committed hematopoietic progenitor cells. Researchers also revealed an inversely proportional association within ALDHbr cells dose and “hematopoietic engraftment time”. In this regards, Vassalli (2019) claimed that CD34+ subset of ALDHbr cells include hematopoietic cells, around 50% of ALDHbr do not express CD34, which are highly supplemented with MSCs and EPCs. These cells from cord blood or bone marrow express genes, which show proangiogenic role and engaged in angiogenesis (Tomita et al., 2016). These molecules have shown to stimulate tissue repair in limb ischemia and MI. Researcher found that this ALDHbr subpopulation from skeletal muscle cells is supplemented in myoblast progenitors, which engraft in a highly effective way through transplantation into skeletal muscle in vivo. The same subpopulation derived from human breast reduction sample is supplemented with multipotent cells, which produces uncommitted, luminal, myoepithelial and mixed colonies. This subpopulation from terminal duct cell or central acinar of adult mouse’s pancreas showed expression of early embryonic pancreas market, which further produces endocrine cells able to secrete insulin. Transplantation of these cells into mouse embryos showed their contribution to both endocrine and exocrine lineages in growing pancreas (Pors & Moreb, 2014). Similarly, ALDHbr progenitor cells from prostatic epithelial cell expressed stem cell antigen-1 and produced prostatic tissue with high efficiency. The researchers also showed that ALDHbr CD34+ cells derived from human cardiac atrial appendage tissue gives rise to mature cardiac myocytes in vivo and in vitro. These results supported the potential use of ALDHs as a marker in variety of normal tissue, which are enriched with the progenitor and stem cells (Vassalli, 2019).
Aldehyde dehydrogenases (ALDHs) as a marker in cancer stem cells
ALDH is widely used as a functional marker in different types of tumor cells. Ma and Allan (2010) highlighted that ALDH superfamily involves the key detoxifying enzymes, which are involved in oxidation of intracellular aldehydes. Activity of these intracellular aldehyes was initially found to be enhanced in neural stem cells, hematopoietic cells and progenitor cells. Vassalli, (2019) showed that compared to ALDH- cells, ALDH+ breast cancer cells in immunocompromised mice had enhanced tumor-initiating ability. In different types of cancers, ALDHbr cells showed stem-like traits, including “clonogenic growth, self-renewal capability tumor-initiating capability and resistance to drugs”. Cytotoxic chemotherapeutic agents are targeting rapidly dividing cells; but, cancer stem-like cells usually remain in quiescent state in the cell cycle, which is one key reason for failure of these treatments to curb cancer in some patients (Cojoc et al., 2015). Further, expression of ABC transporters through these cells can lead to drug resistance. Existing evidences indicate that after chemotherapy, surviving tumor tissue consist high number of cancer stem cells, including ALDHbr. These cells have tumor-initiating ability and found in a range of cancer types. Comparative studies have showed that ALDHbr have higher clonogenic and tumorigenic capacity compared to ALDHdim cells. In different cancers, presence of ALDHbrcells is linked with poor outcomes, but some studies have identified these as independent prognostic factor. Overall, the abundance and tumor-initiating capability of these cells made it to be used as a specific marker for cancer.
ALDH and oxidative stress
In living organisms, biological reactions are continuously producing reactive oxygen species (ROS), which could enhance oxidative stress, if not handled properly, causing alteration of DNA and proteins. Oxidative stress induces lipid peroxidation of phospholipids, generating 200 types of reactive aldehydes. Through metabolizing aldehydes, ALDH can assuage oxidative stress. For instance, ALDH2 and ALDH1A1 can metabolize 4-HNE to 4-hydroxynon-2-enoic acid. Vassalli (2019) found ALDHbr human skeletal myoblasts are able to resist toxic effects hydrogen peroxide in vitro, which strengthens its role in protecting cell against oxidative stress. Cancer stem cells often show low oxidative stress compared to differentiated tumor cells, because of abnormal cell metabolism. For instance, ALDHbr cells derived from ovarian cell carcinoma displayed cancer stem cell cells consisted low level of ROS. As chemotherapeutics and radiation generated enhanced level of oxidative stress in malignant cells, using the “ROS scavenging” capability of ALDH can guard these cells beside the therapeutic procedures through upholding low level of ROS and low DNA damage (Tomita et al., 2016).
Aldehyde dehydrogenases (ALDHs) its potential as therapeutic target in cancer stem cells
The currently available cancer treatments include surgery, chemotherapy and radiation, which are efficient in getting rid of primary tumor. However, several patients are reported with disease recurrence and metastasis. The secondary tumors are mostly accountable for most of the fatality associated with cancer in US. The cancer stem cells are usually extremely resistant to anticancer treatments, which are believed to be accountable for most tumor relapse and metastasis (Tomita et al., 2016). Recent studies are indicating that treatment of malignant cells with X-rays of chemotherapeutic drugs have significant potential to enhance stem cell markers expression, which are associated with stemness characteristics in surviving cells. Such findings are indicating that cancer stem cells are hindering successful resolution of tumor, while imposing threat to survival of the patients. In this context, it has become crucial to find new drugs able to kill these stem cells or stimulate their differentiation, forming normal tumor cells, which can be eliminated through available treatments (Clark & Palle, 2016). ALDHs are expressed in normal stem cells and cancer stem cells and regulate differentiation and maintenance of these cells, which makes it an interesting therapeutic target. The enzyme is important marker for detecting tumor severity, survival and resistance. Although studies have been performed with drugs regulating ALDH, but further work is required for developing efficient chemotherapeutic drugs for improving patient survival. In another study by Cojoc et al., (2015) shed light on further work on ALDH for its use as therapeutic target. The researchers found that ALDH1A1 gene expression is controlled by WNT signaling pathway, which is co-occurring with ?-catenin expression in prostate tumor specimens. They successfully shown that inhibiting WNT pathway can reduce ALDH(+) tumor progenitor population, along with radiosensitization of malignant cells. Therefore, their findings suggested that ALDH(+) cells are contributing to radioresistance; thus, molecular targeting of this gene can increase effectiveness of radiotherapy.
Role of Aldehyde dehydrogenases (ALDHs) in cancer drug resistance
Increasing number of studies are suggesting significant role of ALDHs in developing drug resistance among cancer patients, especially, when administered in high dosage. However, the exact mechanism is yet to be investigated thoroughly. Considering the biological function and mechanisms of the enzyme in acetaldehyde metabolism and oxidative stress, it is noted that usually this enzyme is considered as detoxification enzyme that is crucial for protecting organisms against different harmful aldehydes. ALDH1A1 and ALDH3A1 is offering cellular protection against cytotoxic drugs (Cojoc et al., 2015). Two decades ago, it was noted that hematopoietic and leukemic stem cells with ALDH activity are resistant to alkylating agent and cyclophosphamide. Cytosolic ALDH1A1 and ALDH2A1 are capable of converting activated cyclophosphamide and 4- hydroperoxycyclophosphamide into inactive excretory product “carboxyphosphamide”. As a result, it causes drug resistance, along with radiation resistance (Januchowski, Wojtowicz & Zabel, 2013).
Previous studies on this phenomenon primarily described the chemo-resistant role of ALDHs in a CPA resistant L1210 leukemia cell line. The researcher revealed that elevated level of ALDH activity is seen in this cell line and treating those cells with disulfiram, which is an ALDH inhibitor, kept the resistant phenotype of cell to CPA (cyclophosphamide). A following research further confirmed the role of ALDH in mediating CPA resistance in medulloblastoma. Another similar study by Ibrahim et al., (2018) highlighted that the ALDH activity mediated inhibition can sensitize cancer stem cells to drugs including CPA. Therefore, ALDH1A1 and ALDH3A1 were reported to be inactive CPA analougues. Diverse mechanisms could add in the resistance development. For instance, cancer stem cells are slow-proliferating cells and therefore resist drugs are mainly developed for targeting rapidly growing and diving cells (Januchowski, Wojtowicz & Zabel, 2013). Further, elevated expression of ABC transporters might protect cancer stem cells from elevated level of drugs. Finally, high expression of ALDH is prone to be associated with detoxifying and metabolic mechanisms, which supports its role as chemo-protecting enzyme.
Several investigations have shown that tumors comprise of a heterogeneous cell population, including mass stem cells and tumor cells that initiate malignant cancer (CSCs) (Dehghan Harati, Rodemann & Toulany, 2019). CSCs are able to initiate and auto-renovate the tumor and can lead to large numbers of non-tumor cells through differentiation. In addition, one of the fundamental causes for tumors and therapeutic resistance was proposed as the CSCs. Specific surface markers, including CD44, CD24, or the activity of high aldehyde dehydrogenase 1 (ALDH1) enzymes may be used to separate CSCs from tumor cell subpopulations (Dehghan Harati, Rodemann & Toulany, 2019). The ALDH family is made up of 19 isoform proteins. Among them, ALDH1's enzyme activity is a good predictor for poor overall survival. ALDH1, by the endogenous and exogenous aldehydes oxidation into the carboxylic acids they correspond to, participates in intracellular detoxification (Lynam-Lennon, et al., 2017). The reduced susceptibility to ionised radiation is documented in both mesenchymal stem cells and cancer stem cells (Kamble, et al., 2021). The decreased CSC radiation treatment (RT) sensitivity in many cancer patients can in particular severely impair RT effectiveness (Lynam-Lennon, et al., 2017). Thus, numerous ongoing researches have been undertaken with regard to the underlying mechanisms of the modified radiation reaction of cancer stem cells. ALDH-1 inhibition has demonstrated that breast CSCs is less resistant to radiation and chemotherapy treatment (Kamble, et al., 2021). The targeting in combination with doxorubicin or paclitaxel treatment of ALDH activity in breast CSCs of all transretinoic acid (ATRA) or diethylaminobenzaldehyde (DEAB) and radiation dramatically decreased cell survival comparing to chemotherapy and radionuclide treatment alone (Lynam-Lennon, et al., 2017).
Potential of Aldehyde dehydrogenases (ALDHs) in drug discovery
As a leading type of cancer therapy, selective kinase inhibitors have evolved and several such medicines are now regularly utilized for advanced-stage illness (Yang, et al., 2018). However, their clinical advantage is generally short-lived, because drug resistance after treatment response acquires relatively rapidly (Huddle, et al., 2018). Preclinical and clinical evidence have accumulated indicates a varied reaction to treatment of inherently drug-resistant tumour cells, such as cancer stem cell, within an underpopulation (Li, et al., 2020). In CSCs of many cancer types, Elevated ALDH1A1 is found. ALDH proteins govern aldehyde oxidation to corresponding acids and a drug-resistant characteristics are postulated to ALDH1-positive tumour cells via ALDH-mediated detoxification of hazardous aldehyde intermediates generated in cancer cells treated with specific chemical agents (Li, et al., 2020). The human aldehyde dehydrogenase gene (ALDH) encodes 19 isozymes which are metabolised to their relevant carboxylic acid derivatives by reactive aldehydes (Huddle, et al., 2018). A range of illnesses, including malignancies, have been related with high biological activity of ALDHs (Wang, et al., 2018). The over-expression of various ALDHs, particularly ALDH1A1, corresponds with poor prognosis and aggression and is associated to drug resistance in conventional cancer treatment, in a variety of cancer stem cells (CSC's) and malignancies (Huddle, et al., 2018). Evidence obtained through the use of non-specific ALDH inhibitors and silencing methods for siRNA shows that ALDH1A1 is not only a biomarker and predictor of cancer stem cells, it also plays an essential part in the biology of tumours and cancer stem cells (Wang, et al., 2018).
ALDH1A1-deficient animals have also been reported to show substantial reduction in fasting glucose levels as well as in reduced generation of hepatic glucose and liver triacylglycerol (Huddle, et al., 2018). In addition, the enhanced synthesis of retinal acid with CD14+ macrophages in connection with local ALDH1A1 activation was demonstrated to contribute towards Crohn's disease patients' inflammatory phenotype (Lei, et al., 2019). These findings imply that ALDH1A1 inhibits enzyme activities as well as for cancer, obesity, diabetes, inflammation, novel treatment possibilities (Lei, et al., 2019). For example, a cautious approach to possible new cancer therapies and illnesses is the identification of novel ALDH small molecules such as ALDH1A1 inhibitors with acceptable pharmaceutical characteristics and selective profiles (Lei, et al., 2019). Furthermore, the use of such inhibitors will help researchers get a better understanding under pathophysiological and physiological circumstances of the activity of this enzyme (Wang, et al., 2018).
Potential opportunities of exploiting Aldehyde dehydrogenases (ALDHs) expression for patient benefit
Each individual or organism has been exposed to various harmful chemicals over its lifetime. Some are endogenously produced and some accumulate after dietary intake or environmental exposure (Ueta, et al., 2017). Complete human genome and expression analyses show that 19 ALDH genes with a wide variety of tissue and substratum expression are present. The important role of the ALDH2 in the metabolism of ethanol is better known (Ueta, et al., 2017). We know less about ALDH2, which likewise provides significant protective enzyme activity against those hazardous chemicals, as the various additional short-chain aliphatic aldehydes and some polycyclic and aromatic aldehydes. Such as 4 HHE (4-HNE) and malondialdehysis (MDA) along with environmentally friendly aldehys, ALDH2 plays a vital rôle in oxidising endogenous aldehydes derived from the lipid peroxidation under oxidatives (De Angelis, Francescangeli & Zeuner, 2019). Inhibition of alcohol consumption by ALDH2 has a long history preceding the introduction of molecular medicines in current pharmacological treatments (De Angelis, Francescangeli & Zeuner, 2019). The traditional Chinese pharmaceutical kudzu (Pueraria lobata) has utilized its antidipsotropic action for more than 1,000 years. The two active isoflavone known in the root and flowers of Kudzu are Daidzin and daidzein (Andreadou, et al., 2019).
Only a few tiny molecular activators have been discovered for metabolic enzymes. One example is a family of new aldehyde dehydrogenase activator chemicals known as Aldas (Andreadou, et al., 2019). An in vitro- and in vivo catalytic activity for ALDH2 was discovered to increase the ALDH2 catalytic activity by a class of isozyme-specific agonists Alda-1 [N-(1, 3-benzodioxol-5-ylmethyl)-2, 6-dichlorobenzamide, MW 324] (Andreadou, et al., 2019). Aldehydes may spread rapidly across cell membranes, and create macromolecular adducts, including proteins, DNA and lipids, which normally regulate or impair their function. During oxidative stress as results of lipid peroxidation, reactive aldehydes are ready to develop (Turdo, et al., 2020). A method is therefore critical in order to protect tissues and cells from damage to these extremely diffuse and toxic aldehydes. In conditions of severe aldehyde stress, quicker metabolism and the inactivation of these hazardous aldehydes may ameliorate at least part of the conditions. During oxidative stress as results of lipid peroxidation, reactive aldehydes are ready to develop (Turdo, et al., 2020). A method is therefore critical in order to protect cells and tissues from damage to these extremely diffuse and toxic aldehydes. In conditions of severe aldehyde stress, quicker metabolism and the inactivation of these hazardous aldehydes may ameliorate at least part of the conditions (Fouda, et al., 2020). Since various human illnesses have been connected with oxidative stress, the increase of ALDH2 catalytic activity can offer a new and efficient way to minimise cell-organ dysfunction causing oxidative stress, and thus to improve human health (Fouda, et al., 2020).
The mitochondrial ALDH2 quickly became a critical enzyme for protecting the heart from oxidative stress, out of the 19 distinct ALDH isozymes expressed in the body. While protein downstream of kinase C type ? (?PKC) activation originally found ALDH2, the protein partners of ?PKC include intracellular ion channels. ?PKC has several protein partners (Zhang, et al., 2020). Furthermore, proteomic research shows that ?PKC have numerous intracellular protein partners in cardioprotection models. A more precise treatment goal is to be targeted at ALDH2 in place of ?PKC. Evidence shows that enhanced detoxification of reactive aldehydes such as 4-HNE is protective of acute, like, chronic and ischemia, as cardiovascular illnesses, has been improved using experimental methods employing both genetic over expression and pharmacological activation of ALDH2 (Zhang, et al., 2020). Epidemiological studies have also shown the cardioprotective benefits of ALDH2 that people with a point mutation in the ALDH2 gene (ALDH2*2) leading to a significant decrease in enzyme activity are more prone to heart disease and related morbidity (Kimura, Yokoyama & Higuchi, 2019). The majority is caused by excessive production of a reactive oxygen species, leading to an exacerbation of peroxidation of polyunsaturated fatty acids, such as the arachidonic acid, linoleic acid, and cardiovascular present in biological membranes (Kimura, Yokoyama & Higuchi, 2019). The effect of this damage caused by the heart is often excessive. Subsequently, these lipid hydroperoxides may produce hazardous secondary end products as malondialdehyde and 4-HNE. As stated earlier, 4-HNE is a highly reactive carbonyl molecule that binds easily with histidine, cysteine, and lysine waste and produces adduct protein by a Michael addition or base Schiff reaction (Panisello-Roselló, et al., 2018).
Brain is a high-fatty organ that has a comparatively high level of oxygen content in its lipid bilayer and is rich in poly-unsaturated fatty acid. The major characteristics of the ageing, memory loss, cognitive decay and neurodegeneration are oxidative stress-induced lipe peroxidation, mitochondrial failure, and the accumulation of aldehyde compounds (Deza-Ponzio, et al., 2018). Based on animal research, human clinical results and epidemiological investigations, the function of ALDH2 and aldehydic load in many neurodegenerative disorders has been proposed (Panisello-Roselló, et al., 2018). ALDH2 is a well-known component of 19 different human aldehyde dehydrogenases. Many of what we learned of ALDH2 was due to an interest in the metabolism of ethanol (Deza-Ponzio, et al., 2018). But more recent study shows that this enzyme presents an essential protection against oxidative stress and increased aldehyde burden, which thus contributes to better health (Jaiswal, 2017). Increased aldehyde loads are associating several human illnesses. Aldehydes appear to play a key role in pathologizing a wide range of human illnesses (Jaiswal, 2017).
Potential obstacles of exploiting Aldehyde dehydrogenases (ALDHs) expression for patient benefit
A very large range of biological activity is seen in aldehyde dehydrogenases. ALDH is critical to the production of retinoic acid and to the metabolism of a GABA neurotransmitter as a major regulator of the development of vertebrates. In the field of alcohol metabolism through aldehyde detoxification and for cellular homeostasis, dehydrogenase enzyme activity of ALDH is significant from the toxicological point of view by removing reactive, lipid peroxidation-based aldehydes (Li, Ma & Wong, 2018). However, increased ALDH activity has been shown to interact with some chemical therapies. ALDHs can also operate as esterases and activities without enzymes such as plant osmotic stress reduction, macromolecular bonding such as testosterone and cholesterol and mammalian cornea protection from ultraviolet radiation (Li, Ma & Wong, 2018). ALDH genetic polimorphisms are related to a number of medical conditions, among which are Sjögren-Larsson syndrome, hyperprolinemia type 2 and pyrdoxine-dependent seizures, hyperammonemia, ?-hydroxybutyric aciduria and alcohol diseases, which are responsible for decreasing enzyme activity (Dinavahi, et al., 2019). In the past, ALDH's well-established involvement in the metabolism of alcohol has prompted research into ALDH inhibitors. Acetaldehyde accumulation following the ingestion of ethanol has uncomfortable physiological consequences, including face flushing, nausea and tachycardia (Dinavahi, et al., 2019). In individuals with a genetic variation that provides decreased activity of ALDH2, the enzyme responsible for the effective digestion of acetaldehyde, this condition, called an alcohol flushing syndrome, is often found (Li, et al., 2020). This observation led to the original development as antidipsotropic or alcohol-aversive medicines of selective ALDH2 inhibitors. The reasons for the creation of the selective inhibitors of the particular isozymes become increasingly obvious as our understanding of the roles of the many ALDH isozymes in the conditions of illness continues to increase (Li, et al., 2020). At a minimum, the availability of such inhibitors would enable the supposed roles of isozymes to be verified. Optimum use would be made of these inhibitors for treating illness conditions that include ALDH activity in pathophysiology.
The development of chemotherapeutic drug resistance is a significant clinical problem for cancer therapy. Although most advanced patients react to first chemotherapy, the majority is recurrent within 5 years of treatment; 1/3 is inherently resistant (Gelardi, et al., 2021). CSCs, a small group of carcinogenic cells, are now widely acknowledged to be chemotherapy resistant and hence encourage cancer recurrence as a source of tumor cells. High ALDH activity is a major bio-chemical characteristic of CSCs, as demonstrated in several recent studies that reveal the deep tumor-related potential of ALDH-expressing cells (Gelardi, et al., 2021). Moreover, increased activity of ALDH1A1 and ALDH3A1 isozymes has been linked to the development of resistance to oxazaphosphoric medication, such as cyclophosphamide and procarbazine, from tumour cell populations. ALDH's participation in the resistance mechanism is supported by the fact that ALDH activity is reduced by increasing the potential of these medicines (Skrott, et al., 2019). On the basis of those observations, the novel pharmaceutical objectives for the more effective treatment of cancer have been presented with ALDH isozymes. Novel inhibitors of ALDH, especially ALDH 1A1 and ALDH 3A1, are being produced and evaluated as new drugs (Skrott, et al., 2019). Although suppression of ALDH isozyme is a potentially successful strategy to treating disease, the fact that a build-up of research indicates that activation of ALDH might have positive effects in some illnesses or therapeutic situations is noteworthy (Park, et al., 2021). ALDH2 can be crucial for the protection of ischemic myocardium as well as for its major function in the vasodilator measures of nitroglycerine. Alda-1 decreases the degree of infarction-induced damage by prevention of ALDH2 inaction through ischemia-related 4-hydroxy-2-nonenal (Park, et al., 2021). Fanconi patients are more sensitive to damage of the DNA caused by acetaldehyde. Because the ALDH2 is an essential factor in the metabolization of hazardous aldehydes, the use of an ALDH activator in such individuals is acceptable to hypothesize (Dinavahi, et al., 2019). The necessity for deeper knowledge of the role of each isozyme in certain diseases is emphasised by the fact that ALDH enzymes can be activated or inhibited depending on particular diseases or therapeutic circumstances (Dinavahi, et al., 2019). This information allows predicting the benefits and damages of any inhibitors or activators which have been discovered.
Conclusion
Several investigations have shown that tumors comprise of a heterogeneous cell population, including bulk tumor cells and stem cells that initiate malignant cancer (CSCs) CSCs are able to initiate and auto-renovate the tumor and can lead to large numbers of non-tumor cells through differentiation. Evidence obtained through the use of non-specific ALDH inhibitors and silencing methods for siRNA shows that ALDH1A1 is not only a biomarker and predictor of cancer stem cells, it also plays an essential part in the biology of tumors. ALDH2 plays a vital role in oxidising endogenous aldehydes derived from the lipid peroxidation under oxidatives. Inhibition of alcohol consumption by ALDH2 has a long history preceding the introduction of molecular medicines in current pharmacological treatments. The mitochondrial ALDH2 quickly became a critical enzyme for protecting the heart from oxidative stress. Aldehydes appear to play a key role in pathologizing a wide range of human illnesses. ALDH2 is a well-known component of 19 different human aldehyde dehydrogenases. Increased ALDH activity has been shown to interact with some chemical therapies. Drugs that block the activity in the body's immune system ALDH could be used to treat a number of conditions including diabetes and heart disease. The development of chemotherapeutic drug resistance is a significant clinical problem for cancer therapy. Novel inhibitors of ALDH, especially ALDH 1A1 and ALDH 3A1, are being produced and evaluated as new drugs. They could have positive effects in some illnesses or therapeutic situations.
Reference List
Alison, M. R., Guppy, N. J., Lim, S. M., & Nicholson, L. J. (2010). Finding cancer stem cells: are aldehyde dehydrogenases fit for purpose?. The Journal of pathology, 222(4), 335-344.
Andreadou, I., Cabrera-Fuentes, H. A., Devaux, Y., Frangogiannis, N. G., Frantz, S., Guzik, T., ... & Hausenloy, D. J. (2019). Immune cells as targets for cardioprotection: new players and novel therapeutic opportunities. Cardiovascular research, 115(7), 1117-1130.
Clark, D., & Palle, K. (2016). Aldehyde dehydrogenases in cancer stem cells: potential as therapeutic targets. Annals Of Translational Medicine, 4(24), 518-518. doi: 10.21037/atm.2016.11.82
Cojoc, M., Peitzsch, C., Kurth, I., Trautmann, F., Kunz-Schughart, L., & Telegeev, G. et al. (2015). Aldehyde Dehydrogenase Is Regulated by ?-Catenin/TCF and Promotes Radioresistance in Prostate Cancer Progenitor Cells. Cancer Research, 75(7), 1482-1494. doi: 10.1158/0008-5472.can-14-1924
De Angelis, M. L., Francescangeli, F., & Zeuner, A. (2019). Breast cancer stem cells as drivers of tumor chemoresistance, dormancy and relapse: new challenges and therapeutic opportunities. Cancers, 11(10), 1569.
Dehghan Harati, M., Rodemann, H. P., & Toulany, M. (2019). Nanog signaling mediates radioresistance in ALDH-positive breast cancer cells. International journal of molecular sciences, 20(5), 1151.
Deza-Ponzio, R., Herrera, M. L., Bellini, M. J., Virgolini, M. B., & Hereñú, C. B. (2018). Aldehyde dehydrogenase 2 in the spotlight: The link between mitochondria and neurodegeneration. Neurotoxicology, 68, 19-24.
Dinavahi, S. S., Bazewicz, C. G., Gowda, R., & Robertson, G. P. (2019). Aldehyde dehydrogenase inhibitors for cancer therapeutics. Trends in pharmacological sciences, 40(10), 774-789.
Fouda, A. Y., Eldahshan, W., Narayanan, S. P., Caldwell, R. W., & Caldwell, R. B. (2020). Arginase pathway in acute retina and brain injury: Therapeutic opportunities and unexplored avenues. Frontiers in pharmacology, 11, 277.
Gasparetto, M., & Smith, C. (2017). ALDHs in normal and malignant hematopoietic cells: Potential new avenues for treatment of AML and other blood cancers. Chemico-Biological Interactions, 276, 46-51. doi: 10.1016/j.cbi.2017.06.020
Gelardi, E. L., Colombo, G., Picarazzi, F., Ferraris, D. M., Mangione, A., Petrarolo, G., ... & Garavaglia, S. (2021). A selective competitive inhibitor of aldehyde dehydrogenase 1A3 hinders cancer cell growth, invasiveness and stemness in vitro. Cancers, 13(2), 356.
Huddle, B. C., Grimley, E., Buchman, C. D., Chtcherbinine, M., Debnath, B., Mehta, P., ... & Larsen, S. D. (2018). Structure-based optimization of a novel class of aldehyde dehydrogenase 1A (ALDH1A) subfamily-selective inhibitors as potential adjuncts to ovarian cancer chemotherapy. Journal of medicinal chemistry, 61(19), 8754-8773.
Ibrahim, A. I., Sadiq, M., Frame, F. M., Maitland, N. J., & Pors, K. (2018). Expression and regulation of aldehyde dehydrogenases in prostate cancer. Journal of Cancer Metastasis and Treatment, 4, 1-17.
Jaiswal, M. K. (2017). Therapeutic opportunities and challenges of induced pluripotent stem cells-derived motor neurons for treatment of amyotrophic lateral sclerosis and motor neuron disease. Neural regeneration research, 12(5), 723.
Januchowski, R., Wojtowicz, K., & Zabel, M. (2013). The role of aldehyde dehydrogenase (ALDH) in cancer drug resistance. Biology assignment Biomedicine & Pharmacotherapy, 67(7), 669-680.
Kamble, D., Mahajan, M., Dhat, R., & Sitasawad, S. (2021). Keap1-Nrf2 Pathway Regulates ALDH and Contributes to Radioresistance in Breast Cancer Stem Cells. Cells, 10(1), 83.
Kimura, M., Yokoyama, A., & Higuchi, S. (2019). Aldehyde dehydrogenase-2 as a therapeutic target. Expert opinion on therapeutic targets, 23(11), 955-966.
Lei, H. M., Zhang, K. R., Wang, C. H., Wang, Y., Zhuang, G. L., Lu, L. M., ... & Zhu, L. (2019). Aldehyde dehydrogenase 1A1 confers erlotinib resistance via facilitating the reactive oxygen species-reactive carbonyl species metabolic pathway in lung adenocarcinomas. Theranostics, 9(24), 7122.
Li, B., Yang, K., Liang, D., Jiang, C., & Ma, Z. (2020). Discovery and Development of Selective Aldehyde Dehydrogenase 1A1 (ALDH1A1) Inhibitors. European Journal of Medicinal Chemistry, 112940.
Li, S. S., Ma, J., & Wong, A. S. (2018). Chemoresistance in ovarian cancer: exploiting cancer stem cell metabolism. Journal of gynecologic oncology, 29(2).
Lynam-Lennon, N., Heavey, S., Sommerville, G., Bibby, B. A., Ffrench, B., Quinn, J., ... & Maher, S. G. (2017). MicroRNA-17 is downregulated in esophageal adenocarcinoma cancer stem-like cells and promotes a radioresistant phenotype. Oncotarget, 8(7), 11400.
Ma, I., & Allan, A. (2010). The Role of Human Aldehyde Dehydrogenase in Normal and Cancer Stem Cells. Stem Cell Reviews And Reports, 7(2), 292-306. doi: 10.1007/s12015-010-9208-4
Moreb, J. S., Ucar-Bilyeu, D. A., & Khan, A. (2017). Use of retinoic acid/aldehyde dehydrogenase pathway as potential targeted therapy against cancer stem cells. Cancer chemotherapy and pharmacology, 79(2), 295-301.
Muralikrishnan, V., Hurley, T. D., & Nephew, K. P. (2020). Targeting aldehyde dehydrogenases to eliminate cancer stem cells in gynecologic malignancies. Cancers, 12(4), 961.
Panisello-Roselló, A., Lopez, A., Folch-Puy, E., Carbonell, T., Rolo, A., Palmeira, C., ... & Roselló-Catafau, J. (2018). Role of aldehyde dehydrogenase 2 in ischemia reperfusion injury: An update. World journal of gastroenterology, 24(27), 2984.
Park, H. H., Park, J., Cho, H. J., Shim, J. K., Moon, J. H., Kim, E. H., ... & Kang, S. G. (2021). Combinatorial Therapeutic Effect of Inhibitors of Aldehyde Dehydrogenase and Mitochondrial Complex I, and the Chemotherapeutic Drug, Temozolomide against Glioblastoma Tumorspheres. Molecules, 26(2), 282.
Pors, K., & Moreb, J. S. (2014). Aldehyde dehydrogenases in cancer: an opportunity for biomarker and drug development?. Drug discovery today, 19(12), 1953-1963.
Raha, D., Wilson, T. R., Peng, J., Peterson, D., Yue, P., Evangelista, M., ... & Settleman, J. (2014). The cancer stem cell marker aldehyde dehydrogenase is required to maintain a drug-tolerant tumor cell subpopulation. Cancer research, 74(13), 3579-3590.
Skrott, Z., Majera, D., Gursky, J., Buchtova, T., Hajduch, M., Mistrik, M., & Bartek, J. (2019). Disulfiram’s anti-cancer activity reflects targeting NPL4, not inhibition of aldehyde dehydrogenase. Oncogene, 38(40), 6711-6722.
Sládek, N. E., Kollander, R., Sreerama, L., & Kiang, D. T. (2002). Cellular levels of aldehyde dehydrogenases (ALDH1A1 and ALDH3A1) as predictors of therapeutic responses to cyclophosphamide-based chemotherapy of breast cancer: a retrospective study. Cancer chemotherapy and pharmacology, 49(4), 309-321.
Tomita, H., Tanaka, K., Tanaka, T., & Hara, A. (2016). Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget, 7(10), 11018-11032. doi: 10.18632/oncotarget.6920
Turdo, A., Porcelli, G., D’Accardo, C., Franco, S. D., Verona, F., Forte, S., ... & Stassi, G. (2020). Metabolic escape routes of cancer stem cells and therapeutic opportunities. Cancers, 12(6), 1436.
Ueta, C. B., Gomes, K. S., Ribeiro, M. A., Mochly-Rosen, D., & Ferreira, J. C. (2017). Disruption of mitochondrial quality control in peripheral artery disease: new therapeutic opportunities. Pharmacological research, 115, 96-106.
Vassalli, G. (2019). Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells International, 2019, 1-15. doi: 10.1155/2019/3904645
Wang, R., Yang, L., Li, S., Ye, D., Yang, L., Liu, Q., ... & Li, X. (2018). Quercetin inhibits breast cancer stem cells via downregulation of aldehyde dehydrogenase 1A1 (ALDH1A1), chemokine receptor type 4 (CXCR4), mucin 1 (MUC1), and epithelial cell adhesion molecule (EpCAM). Medical Science Monitor: international medical journal of experimental and clinical research, 24, 412.
Xu, X., Chai, S., Wang, P., Zhang, C., Yang, Y., Yang, Y., & Wang, K. (2015). Aldehyde dehydrogenases and cancer stem cells. Cancer letters, 369(1), 50-57.
Yang, S. M., Martinez, N. J., Yasgar, A., Danchik, C., Johansson, C., Wang, Y., ... & Maloney, D. J. (2018). Discovery of orally bioavailable, quinoline-based aldehyde dehydrogenase 1A1 (ALDH1A1) inhibitors with potent cellular activity. Journal of medicinal chemistry, 61(11), 4883-4903.
Zhang, R., Liu, B., Fan, X., Wang, W., Xu, T., Wei, S., ... & Chen, Y. (2020). Aldehyde dehydrogenase 2 protects against post-cardiac arrest myocardial dysfunction through a novel mechanism of suppressing mitochondrial reactive oxygen species production. Frontiers in pharmacology, 11, 373.